
Photo│Chang Yu-Chen
A Scientist Hooked on the Microscopic World
”The experiment I remember most vividly? It’s the one that scared me the most!” Dr. Lin laughs, recalling her high school biology class. “Whenever my classmates dissected bullfrogs, I’d hang way back and watch from a distance…” We often picture scientists as fearless explorers, but Dr. Lin’s story reminds us that even researchers have subjects they’d rather avoid.
But there are many paths to a scientific career. Dr. Lin, an expert in the biosynthesis of “natural products,” didn’t find her calling amidst bubbling beakers of mysterious “Chemical X.” Instead, her journey started far from the typical lab, in the rural fields of Yilan County, Taiwan.
Growing up surrounded by nature – before the landscape became dotted with numerous farmhouses and guesthouses – gave Lin ample opportunity to connect with the environment. She fondly remembers elementary school science class being her favorite. She devoured the science columns in a popular children’s daily newspaper (國語日報), her eyes wide with curiosity about plants, animals, and insects, and natural phenomena.
Back when she was the age for cartoons, an animated series called “Once Upon a Time… Life” (a French series popular in many countries, including Taiwan, that explained the human body) frequently lit up the Lin family TV. “It depicted the microscopic world inside the human body, personifying different cells,” she explains. “Each episode tackled a theme, like white blood cells fighting off bacterial invaders, or how the body metabolizes sugars and fats after a meal.” Though the memories are distant, recalling the show still brings back her childlike wonder.
”There’s this whole microscopic universe hidden from our eyes, offering incredible room for imagination,” Lin reflects. “It wasn’t until much later, through my research, that I realized how many self-contained systems exist in this micro-world, like the pathways for synthesizing natural products.”
Photo│YouTube
Building Molecules for Survival
So, what exactly are natural products? You could stare at complex chemical structure diagrams that look like an alien language. But Dr. Lin puts it simply: “Think about the caffeine in your coffee, or ginkgo biloba extract people take for memory. These originate from natural products and have specific effects on our bodies.”
These “specific effects” refer to the biological or pharmacological activity of natural products. However, the fungi, bacteria, or plants making them weren’t initially aiming to benefit humans. They produce these compounds primarily to survive in their own environments – maybe to fight off rivals or help out a symbiotic host.
In essence, natural products are the “tools” organisms use to interact – sometimes for mutual benefit, sometimes for warfare. Take okaramine, for instance. It’s a nitrogen-containing alkaloid with a very specific purpose. The name gives a clue: “Okara” is Japanese for the soy pulp left after making tofu or soy milk, and “amine” refers to nitrogen-containing compounds. Scientists first discovered that certain Penicillium fungi growing on okara produced okaramines. Later, chemists found these molecules possess selective insect-killing properties.
In plain English? The fungus growing on the soy pulp makes okaramine to basically shout at competing insects: “This spot is mine! Trespassers will be eliminated!”
This microscopic turf war fascinates chemists and sparks a key question: Can we artificially synthesize these useful natural products? For okaramine, a complete chemical synthesis method hasn’t been cracked yet. To figure it out, you first need to understand how the organism builds the molecule – which atoms it uses and what steps it takes, much like following LEGO instructions.
Thinking Like playing with LEGOs: Assembling Okaramine
Dr. Lin compares natural product research to playing with LEGOs. Atoms like carbon, hydrogen, oxygen, and nitrogen are the individual bricks. Early scientists knew fungi used these “organic” bricks to build impressive “LEGO battleships” (the natural products) to fight insect enemies, but they didn’t know the assembly instructions. So, they tried to replicate the final structure in the lab using the same atomic bricks, guessing at the steps involved.
For over a century, chemists have used “chemical synthesis” – employing highly reactive chemicals, often under high heat or pressure – to try and construct complex molecules.
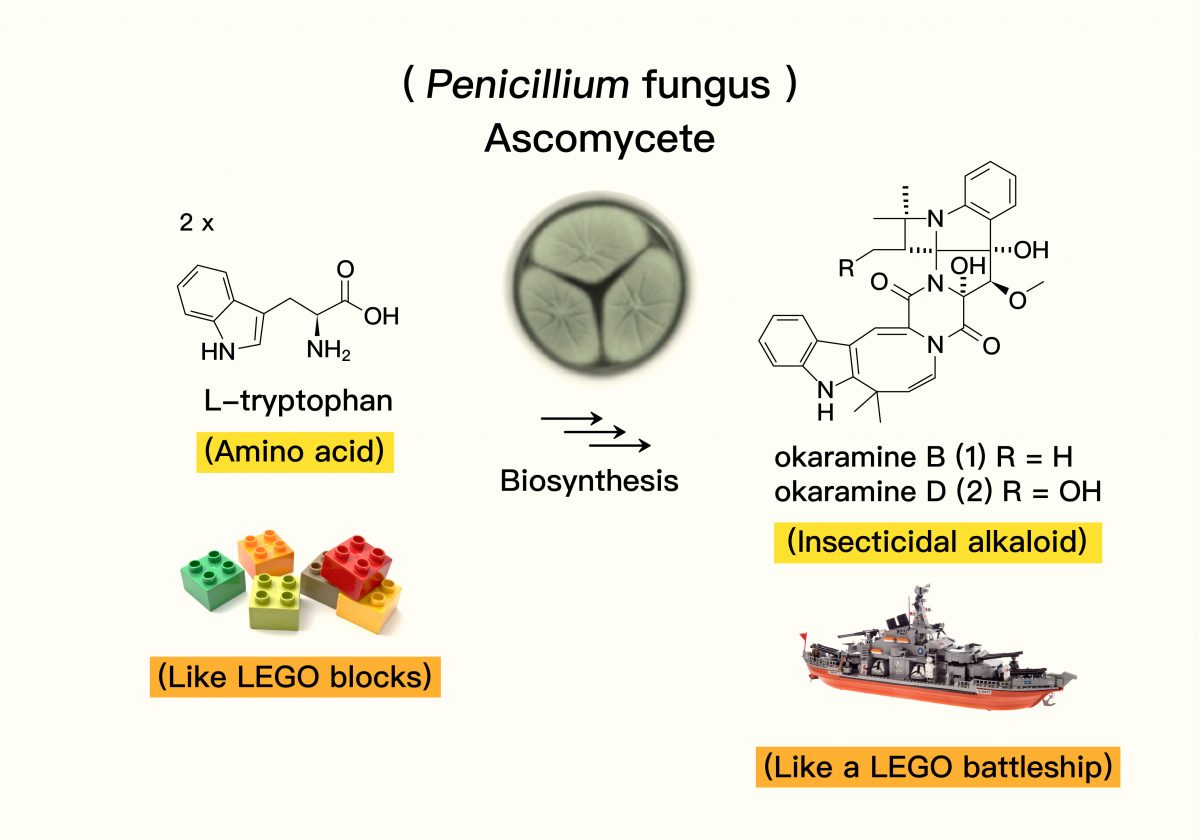
Source: Biosynthesis of Complex Indole Alkaloids: Elucidation of the Concise Pathway of Okaramines. Diagram adapted by: Lin Ting-Hsien and Chang Yu-Chen
But nature has its own elegant toolkit: enzymes. These proteins act as biological catalysts, speeding up specific chemical reactions. DNA holds the blueprints for its enzymes, dictating their functions. Thanks to recent advances in genome sequencing technology, scientists can now peek at nature’s instruction manual, identifying the genes and enzymes involved in biosynthesizing various compounds.
Using a combination of techniques—genome mining, gene knockout (disabling specific genes), heterologous expression (making one organism produce another’s enzymes), and detailed chemical structure analysis—Dr. Lin’s team delves into the microscopic world. They identify the key genes and enzymes responsible for building molecules like okaramine and figure out exactly what role each plays. They then test these enzyme-driven reactions in the lab, mimicking natural conditions (room temperature, normal pressure) in simple water-based solutions containing the necessary starting materials (substrates) and helpers (cofactors).
Cracking the Code: The OkaE Discovery
”Our biosynthesis approach is different from traditional chemical synthesis,” Dr. Lin clarifies. “We don’t need harsh, highly reactive, or toxic chemicals. We want to understand the methods nature actually uses.”
She holds up a colorful ball-and-stick model of okaramine, looking distinctly like connected LEGO pieces. Black spheres represent carbon atoms, blue are nitrogen, and red are oxygen. Her team uncovered a crucial step in okaramine assembly. A specific enzyme, which they named OkaE, acts like a molecular matchmaker. It catalyzes a tricky reaction, joining two carbon atoms (that were previously separate parts of precursor molecules) together, ultimately forming a key four-ring structure within the okaramine molecule.
Trying to snap the model pieces together herself, Lin demonstrates the point: “See? Even by hand, it takes a bit of force to connect these! Inside the fungus, forming this four-ring structure also requires significant energy. Nature uses the OkaE enzyme to make it happen efficiently. And our team was the first to figure that specific step out!” she says with a bright smile.
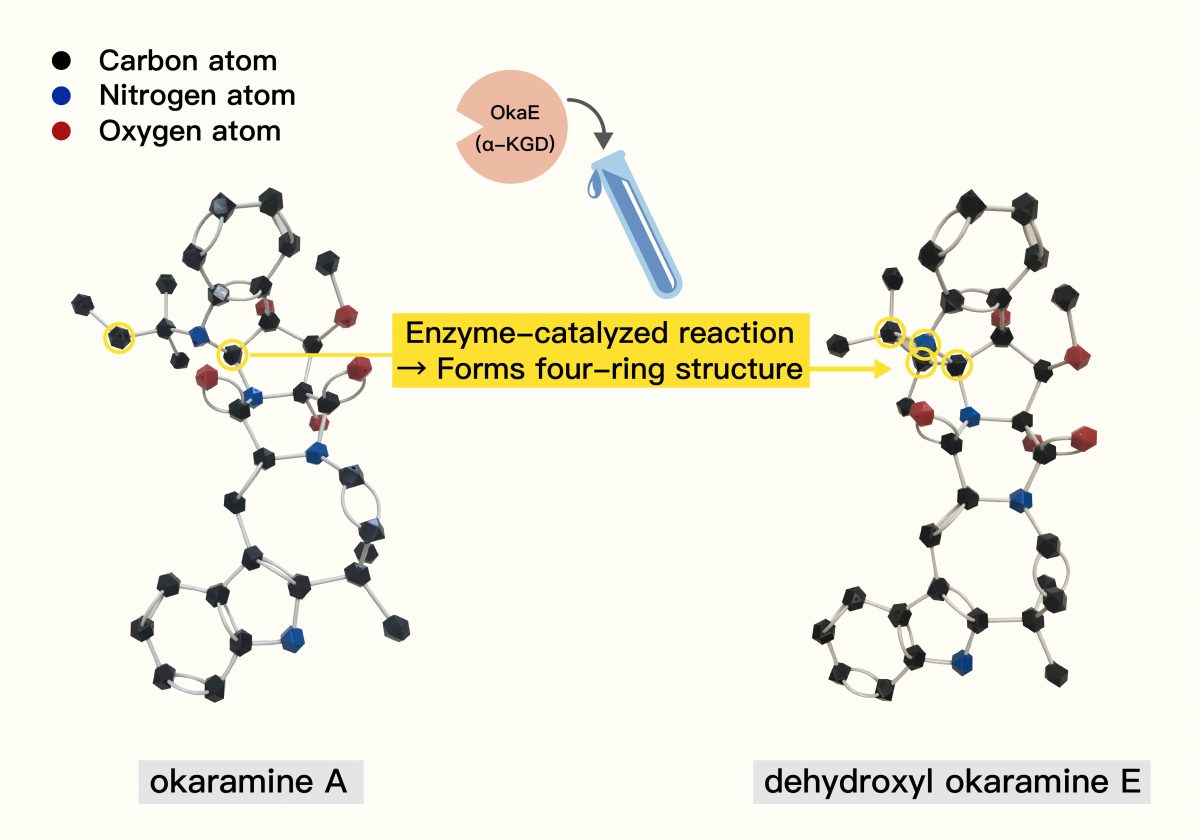
Source: Biosynthesis of Complex Indole Alkaloids: Elucidation of the Concise Pathway of Okaramines. Diagram adapted by: Lin Ting-Hsien and Chang Yu-Chen
Why Study Nature’s Blueprints?
While many natural catalytic enzymes and their biosynthetic pathways remain undiscovered, Dr. Lin finds that part of the allure. “There’s still so much unknown territory,” she says. “And what’s fascinating about this research is… you feel like you’re uncovering fundamental truths. The function of a specific enzyme, confirmed through various experiments, always points to the same conclusion. It’s like discovering a law of nature.”
Another powerful motivation is the potential for developing new medicines. Consider Paclitaxel (Taxol), a vital cancer drug. It’s a natural product originally isolated from the bark of the Pacific yew tree. Later, scientists found they could get a precursor molecule from the needles of the related European yew and use “semi-synthesis” to make Taxol. However, the yew trees produce very little of the compound naturally, creating a major bottleneck for drug development and delaying its availability.
If we can fully understand nature’s recipes – the genes, the enzymes, and the step-by-step pathways – we could potentially use synthetic biology to produce valuable compounds like these more efficiently and sustainably, overcoming natural supply limitations.
But could mass-producing natural compounds artificially harm the environment? Dr. Lin addresses this thoughtfully: “Different natural products have different activities. If we decide to produce a specific compound on a large scale, we must first be very clear about its purpose and potential impact. However, it’s worth remembering that these molecules originate in nature, so nature also possesses corresponding biodegradation mechanisms.”
We often say, “seeing is believing.” But beyond the world visible to our eyes, perhaps on a tree branch right outside your window, a hidden universe teems with microscopic life. And within it, fungi might just be busy playing with their molecular LEGOs, building the next wonder drug or biological tool, molecule by molecule.


